Last updated: October 15, 2020
Article
A Preliminary Report on the Bees of Joshua Tree National Park, with Special Focus on Anthophora Digger Bees
Michael C. Orr, Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese Academy of Sciences, Beijing, P.R. China.
Background
Deserts are extreme environments that force organisms to adapt and compete for resources that are limited in both time and space. By studying organisms that thrive in deserts, we can better our knowledge of how environments drive evolution. Bees are an exceptionally rich study system because of their close relationship with flowers, which are, in deserts, tied to limited and stochastic water resources (Michener 1979, Michener 2007, Minckley et al. 2000).
Despite the challenges that deserts pose, bees attain their greatest species richness in xeric areas, possibly a result of their hypothesized origin in an arid region of Gondwana, where they would have accumulated many strategies for surviving such harsh conditions (Litman et al. 2011, Michener 1979). Regardless of the cause, xerophilic bees exhibit a number of apparent adaptations for desert life, including highly opportunistic emergence times and the ability to wait multiple years to emerge, both of which enable better tracking of local floral resources (Danforth 1999, Hurd 1957, Orr et al. 2016). It has also been suggested that deserts cause a higher degree of floral specialization than is seen in other environments (Minckley et al. 2000).
Floral specialists, or oligoleges, are those bees that consistently use a certain subset of the total flowering plant species available to them (Cane and Sipes 2006, Wcislo and Cane 1996). In deserts, it has been suggested that floral specialists are better able to track their specific floral hosts than generalists can track floral resources overall (Minckley et al. 2000). This seems intuitive when one considers that different plant species use disparate cues for seed germination and flowering times, therefore bees need to adapt to these shifting cues in the same way the plant does (Adondakis and Venable 2004, Jurado and Westoby 1992, Kemp 1983, Tevis 1958). Unfortunately, the floral preferences of many bees remain unknown, and recent reports call into question some past assignments of specialization (Cane and Sipes 2006, Nelson and Griswold 2015, Ritchie et al. 2016, Wilson et al. 2009). Clearly, more work is needed to understand the basic biology of these vital pollinators.
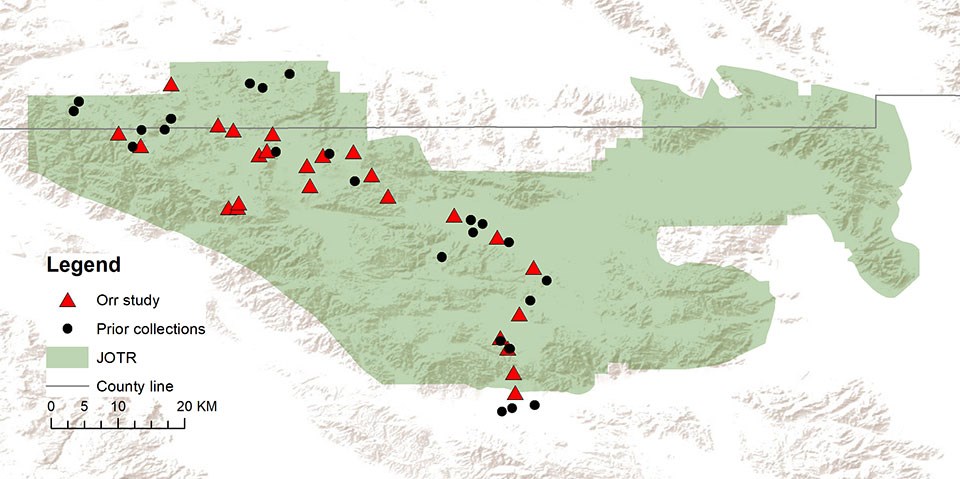
Figure 1. Collection sites within and around JTNP for specimens used to create the inventory for this study. The present-day area of JTNP is indicated as a green polygon. Localities from the present study are given as red triangles and prior collections are given as black circles. County lines are given in gray (San Bernardino County north, Riverside County south). Scale 1:600,000
Situated at the interface of the Mojave and Sonoran Deserts, Joshua Tree National Park (JTNP) is an ideal area for studying desert bee ecology and evolution (Figure 1). Further, studies in protected areas such as this are vital for an informed balance between conservation and recreation. Detailed in this article are data collected during preliminary bee surveys in JTNP, including the documentation of several rare species and significant range extensions. Because the author’s primary research focus is on the xerophilic genus Anthophora (Brooks 1988, Orr et al. 2014), greater focus is given to this group of bees in this article, especially the floral preferences of Anthophora, the Digger Bees. Anthophora (Figure 2) is a large genus of solitary bees, commonly referred to as Digger Bees because nearly all of these bees are strong nest excavators, typically of soil (Brooks 1988). These bees are found worldwide, however, they are clearly more prevalent in arid areas, making JTNP an ideal habitat.

Michael Orr
Figure 2. The Anthophora genus is a diverse group of solitary bees that are known for ground nesting, though many also use banks, hence their common name, Digger Bees. Left: Psorothamnus specialist Anthophora hololeuca was newly detected within JTNP in this study; Center: Anthophora pueblo peeking out of its nest in a sandstone bank; Right: Anthophora urbana, a supergeneralist species, collecting pollen from Ericameria (Asteraceae).
Objectives
- Compile an initial inventory of bees known to occur in Joshua Tree National Park.
- Document exceptionally rare bee species or unusual range extensions.
- Learn more about the floral specialists present in the park, with focus on the genus Anthophora.
|
Family |
Genius |
Source |
|---|---|---|
| Andrenidae | Andrena | Orr |
| Calliopsis | Orr | |
| Macrotera | Orr | |
| Megandrena | Both | |
| Perdita | Both | |
| Pseudopanurgus | Both | |
| Apidae | Anthophora | Both |
| Anthophorulao | Orr | |
| Apis | Both | |
| Centris | Orr | |
| Ceratina | Orr | |
| Diadasia | Orr | |
| Ericocis | Orr | |
| Eucera | Orr | |
| Exomalopsis | Orr | |
| Habropoda | Orr | |
| Melissodes | Orr | |
| Peponapis | Orr | |
| Svastra | Orr | |
| Tertraloniella | Orr | |
| Triepeolus | Orr | |
| Xeromelecta | Orr | |
| Colletidae | Colletes | Both |
| Hylaeus | Orr | |
| Halictidae | Augochlorella | Orr |
| Conanthalictus | Both | |
| Dieunomia | Orr | |
| Dufourea | Both | |
| Lasioglossum | Orr | |
| Nomia | Orr | |
| Xeralictus | Orr | |
| Megachilidae | Anthidiellum | Prior |
| Anthidium | Both | |
| Ashmeadiella | Both | |
| Atoposmia | Both | |
| Coelioxys | Orr | |
| Dianthidium | Both | |
| Hoplitis | Both | |
| Megachile | Both | |
| Osmia | Both | |
| Stelis | Prior | |
| Trachusa | Orr | |
| Melittidae | Hesperapis | Both |
Table 1. List of bee genera known to occur in JTNP. This inventory documented 6 families and 43 genera; importantly, 63% of the genera documented were new for the inventory. “Source” identifies whether the taxon was observed during this study only (Orr), prior studies only (Prior), or both.
Methods
Given the spatial and temporal stochasticity of both precipitation and flowering time, opportunistic sampling was used to target unique or promising locations within JTNP (Figure 1), with special emphasis on documenting the species richness of Anthophora within the park. Sweep net sampling from plants most likely to be visited by bees was conducted. A total of 295 specimens were collected and subsequently deposited in the USDA-ARS National Pollinating Insects Collection (NPIC). During 2015-2016, a total of eight days were spent sampling within the park boundaries, not including those days during which sampling was impossible due to inclement weather or insufficient bloom (>20 days were spent in the park overall). Sampling dates include the following days during spring: 24–25 March 2016 and 19–21 April 2016. Fall collections took place on the following days: 23–25 August 2015. To augment the data collected through field surveys, specimen records with adequate georeferenced localities (Figure 1) from the NPIC were included in the data set for compiling the inventory list (NPIC 2017). All specimens that were located within any 25 km grid within or entering JTNP’s boundaries were included on the inventory (Table 1). Identification of field-sampled specimens was done to the genus or species level then deposited in the NPIC. Among bees, it is not unusual for specimens to be unidentifiable to species because many of them remain undescribed or because identification resources for many groups are entirely lacking.| Family | Genus | Ecological Notes | Reason |
|---|---|---|---|
| Andrenidae | Ancylandrena | 2 | |
| Apidae | Bombus | 2 | |
| Epoeolus | Kleptoparasite | 3 | |
| Holcopasites | Kleptoparasite | 3 | |
| Maratinapis | Rare | 1 | |
| Melecta | Kleptoparasite | 3 | |
| Neolarra | Kleptoparasite | 3 | |
| Neopasites | Rare, Kleptoparasite | 1, 3 | |
| Nomada | Kleptoparasite | 3 | |
| Oreopasites | Kleptoparasite | 3 | |
| Townsendiella | Rare, Kleptoparasite | 1, 3 | |
| Xylocopa | 2 | ||
| Zacosmia | Kleptoparasite | 3 | |
| Halictidae | Agapostemon | 2 | |
| Halictus | 2 | ||
| Protodufourea | Rare | 1 | |
| Sphecodes | Kleptoparasite | 3 | |
| Sphecodosoma | Rare | 1 | |
| Megachilidae | Chelostoma | 2 | |
| Dioxys | Rare, Kleptoparasite | 1, 3 | |
| Heriades | 2 | ||
| Lithurgus | 2 | ||
| Protosmia | 2 | ||
| Melittadae | Melitta | Rare | 1 |
Table 2. List of genera expected to occur in JTNP but missing from the inventory data. Reasons for missing taxa from the current database include, but are not limited to: 1) low abundance; 2) insufficient sampling; or 3) ecological restrictions.
Additional information regarding rarity, distribution, and/or interesting biology and ecology for each taxon was gleaned from public databases, such as DiscoverLife (DL 2017) and Global Biodiversity Information Facility (GBIF 2017); however, the bulk of this information was compiled based on the author’s, and other experts, knowledge of these groups (Michener 2007). For example, in analyzing the inventory produced by this effort, it is important to look at which taxa were expected to be observed, but were not. The absent taxa from our inventory could be a result of many things, but most likely it falls into one of three categories: 1) the taxon is naturally rare on the landscape spatially or temporally, or simply low in numbers (abundance); 2) as a result of insufficient sampling (due to lack of flowering, poor weather conditions, etc); or 3) the biology or ecology of the bee makes them difficult to capture (e.g. kleptoparasites). In an effort to facilitate future collecting efforts, these taxa were identified (Table 2).
Photographs were taken with a Pentax Optio WG-2 and a Nikon D3300 (not all pictures were taken within the park). Any microscope images were taken with a Keyence VHF-500x Digital Microscope. Maps were generated with ArcMap 10.3 as well as ESRI basemaps and shapefiles.
Results
Twenty-five distinct locations were successfully sampled throughout the park in this study, though over 40 were visited (Figure 1). To maximize the time spent collecting, areas adjacent to roads were prioritized. Sites that were visited multiple times include Cottonwood Wash, Key’s View, Quail Springs Valley, Queen Valley, and various stops within the Pinto Basin. In the future, special focus should be given to less accessible southern localities that fall within the Sonoran Desert ecoregion. For the current study, only the two southernmost localities (Figure 1) in the park fell within this ecoregion (as described by Olson et al. (2001)). In addition, future work should focus on areas with unique habitats that were not sampled in this study.
Species richness is a fundamental ecological metric; it refers to the number of taxa found in an area. Within JTNP, it is expected that bee species richness will be high due to its geographic size and habitat diversity. As a comparison, Michener (1979) predicted that about 500 species are expected in the adjacent Palm Springs area; because JTNP is superior in both size and habitat diversity, it seems likely that bee richness in the park is nearer to 600 species or approximately 65 genera. During this study, 295 specimens were collected, representing six families and 41 genera. The complete dataset is based on 459 total specimens, which includes 164 prior collections; it documents six families and 43 genera (Table 1). As discussed earlier, insect inventory studies often have many taxa that cannot be identified to species level, thus discussions about diversity often focus on a broader level, such as the genus or family, as is done in this case.
The field sampling efforts conducted during this project recovered 41 bee genera, the majority of these (27/43, 63%) were new for the park inventory. This means that with only eight days of field collecting, this study nearly tripled the known genera for the park (NPIC 2017; Table 1). Prior efforts had only documented 16 genera, two of which were not observed during this study. For a number of reasons, as mentioned above, some genera are harder to observe in nature. In addition, there can be sampling bias in the field or represented in museum collections as a result of research interests of particular entomologists. For example, the highest percent of shared genera between this collection effort and past efforts was seen in the family Megachilidae (7/11, 64%), which is the group of bees that the NPIC collection manager, Dr. T. Griswold, is an expert on. Interestingly, the two genera that were not documented during this field survey also belonged to this family. It seems likely that further data mining efforts in other institutions will raise the number of taxa found in JTNP, but, more importantly, promoting field collections and insect surveys will certainly add significantly to the inventory of invertebrates for the park.

Michael Orr
Figure 3. Ericrocis lata, one of the more photogenic kleptoparasitic bees collected in JTNP, resting upon a stem of Bebbia juncea. Several additional individuals were also present. This bee invades nests of Centris, of which three species were collected (more are expected).
Despite the current and past inventory efforts, there are a number of expected genera that remain unknown from JTNP (Table 2). There are 24 genera that have been identified as being “likely present” in the park (Michener 2007, NPIC 2017; Table 2) based on a variety of things, such as expert knowledge, known occurrences just outside the park boundary, the presence of good habitat and host plants, and/or low detection rates for the type of bee. The fact that exceedingly common groups are absent from the collection record, such as Agapostemon and Halictus, demonstrates that insufficient sampling effort could account for many of these missing taxa. Conversely, for seven of these genera, the relative rarity of these groups may explain their absences, in other words, these rare bees are hard to find even under ideal conditions. There are also 11 kleptoparasitic genera among the “likely present” taxa that have yet to be collected, compared to only five in the known genera (Figure 3). In this case, the bees are detected infrequently because they are most easily found patrolling for the nest sites that they invade (rather than needing to collect pollen for themselves); in other words, kleptoparasites are not as often found around flowers, where most collections took place in this study (Michener 2007). Additional fieldwork that focuses on capturing these types of bees would certainly yield more taxa for the inventory. In addition, as insect collections across the country continue to database their specimens, it seems likely that these gaps will be filled.
Rare bees, range extensions, and species of special interest
Any attempt to compile an inventory will lead to interesting findings; for example, field collections can lead to the discovery of a new species to science. Or, more commonly, a species is discovered in a place it was unknown prior - this is called a range extension. Rarity is a term that is generally reserved for species that naturally occur in low numbers (low abundance) or have a narrow geographic range. The tricky part about defining rarity for a species is that often there is a paucity of data to pull from to fully explain why there are very few collections of a certain taxon in an area. In other words, rarity might be due to their biology (e.g., restriction to a specific host plant or nesting substrate) or an artifact of insufficient sampling. Below is a list of species pulled from the inventory that are considered rare or of special interest based on the data thus far. They are listed alphabetically by family then species.
Andrenidae (Mining Bees):
Perdita bebbiae: Prior to this study, this extremely rare species was only known from one location: south of Ocotillo, CA, in San Diego County (Timberlake 1956). During this effort, a second specimen was documented from JTNP east of Squaw Tank. In both instances it was collected on Bebbia juncea (sweetbush); this may be its primary or sole host, however, with so few collection records, it would be premature to declare this. If it is limited to Bebbia juncea, floral limitation could not explain its rarity, as Bebbia juncea is both widespread and common throughout the southwestern U.S. and northwestern Mexico (Tropicos 2017). A better explanation for its rarity may be that its nesting habits are unusually restrictive, which would mean the species becomes less abundant away from its nesting habitat.
Perdita mucronata: The biology of this rare species is exceptionally poorly known. There is only one known location for this species in the world: near Cottonwood Springs. Unfortunately, the only collection is from 1963 and it lacks any kind of information about the habitat or associated plants it was found near (Timberlake 1956). It was not observed or collected during the current study.
Apidae (Apid Bees)
Anthophora columbariae: Recently, Kopec and Burd (2017) listed this relatively uncommon species as “declining,” although the exact methods used in this study were not published and a mechanism of decline is not evident. Interestingly, it was found plentifully throughout JTNP (33 specimens from seven sites). Its conservation status remains uncertain (Orr et al. 2018). This species is primarily known from more mild environments in California’s coastal ranges. Two other primarily “coastal” species found in JTNP are Conanthalictus nigricans (Halictidae) and Habropoda tristissima (Apidae). A number of additional species are known from the coastal ranges and higher elevations of the Mojave Desert (especially the Mojave National Preserve), which suggests that the park’s upper elevations might be an important transitional zone between these environments.
Anthophora signata: This uncommon species is one of only two Anthophora known to nest in wood, whereas all but two of the other 400+ taxa in this genus are ground-nesting (Brooks 1988). Fitting for this current inventory study, it was previously recorded nesting in Joshua Trees (Yucca brevifolia). However, according to Brooks (1988), it may also nest in the ground; it may be that this atypical wood-nesting behavior is opportunistic in nature, as related species do not use wood.
Halictidae (Sweat Bees)
Dufourea snellingi: This taxon was originally described in 1980 based on specimens collected from Upper Covington Flat in JTNP. Since then, it has only been reported from a few disjunct locations in the California desert. With so few recorded observations of this species, very little is known about it. This genus contains many floral specialists that use a wide variety of plants, and it seems likely that this species is also a specialist (Michener 2007). Unfortunately, this bee was not observed during this project and the host plant for this species remains unknown.
Megachilidae (Leafcutter and Mason Bees)
Trachusa autumnalis: The distribution of this species reaches over a large area geographically, but is only known from three isolated patches: from Vidal Junction (near the AZ-CA border), Baja California Sur, and now JTNP. This species may specialize on Asteraceae, as it was collected on this family in 2/3 cases (on Bebbia juncea in JTNP), but both Bebbia juncea and Asteraceae in general are common in the desert, meaning this alone could not explain its rarity in collections. One interesting fact about these collections is that they all occurred during late summer and fall (Aug-Oct), when temperatures are high and any flowering that is happening then will be due to monsoonal rain events. Asteraceae includes many summer-blooming plants, so this bee may have evolved to emerge in response to hot summer rains necessary for plant germination. This would easily account for a lack of specimens because summer blooms are hard to track and most people do not think of collecting during the hottest time of the year. As bee collectors generally time their trips with periods of high bloom, avoidance of late summer could bias sampling against species that are specifically active during these times.
| Subgenus | Species | Documentation | Notes |
|---|---|---|---|
| Anthophoroides | californica | Expected, not observed | |
| Lophanthophora | neglecta | Expected, not observed | |
| Micranthophora | columbariae | Both | |
| Micranthophora | curta | Both | |
| Micranthophora | estebana | Both | |
| Micranthophora | petrophila | Both | |
| Mystacanthophora | urbana | Both | |
| Paramegilla | centriformis | Both | |
| Paramegilla | fulvicauda | Both | |
| Anthophoroides | signata | Orr | |
| Lophanthophora | coptognatha | Orr | |
| Lophanthophora | dammersi | Orr | |
| Micranthophora | abroniae | Orr | |
| Micranthophora | hololeuca | Orr | |
| Pyganthophora | vannigera | Orr | |
| Micranthophora | pachyodonta | Prior | |
| Anthophoroides | cinerula | Expected, not observed | Undescribed at time of study |
| Anthophoroides | pueblo | Expected, not observed | Undescribed at time of study |
| Micranthophora | striata | Expected, not observed | Undescribed at time of study |
| Micranthophora | parkeri | Orr | Undescribed at time of study |
| Micranthophora | timerlakei | Orr | Undescribed at time of study |
Table 3. Species of Anthophora found or expected to occur in JTNP. Documentation refers to voucher specimens collected during this study (Orr), prior studies (Prior), or both. “Expected, not Observed” refers to species that are likely present in JTNP, based on nearby observations, but have yet to be documented. Five taxa are new to science and have yet to be described, two of which were first documented during this study.
The Anthophora of JTNP
As with many bees, Anthophora (Digger Bees) attains its highest number of species in deserts (Michener 1979). Almost 60 of the 400 described species of Anthophora occur north of Mexico in the Western Hemisphere, most of which (>75%) reside in the xeric Southwest. Prior to this study only eight species of Anthophora had been documented in JTNP; now, there are a total of 16 unique taxa documented in the park based on a total of 113 Anthophora specimens (Table 3). Six additional species are likely present in JTNP, based on nearby observations, but have yet to be documented. Consequently, total sampling efforts have recovered 73% of the expected Anthophora in JTNP. Of more significance is that two of the 16 documented Anthophora species are undescribed taxa and three of the six “expected” taxa were also undescribed at the time of this study. This is a testament to the incredible invertebrate diversity yet to be discovered in the Desert Southwest: nearly one-quarter of all documented Anthophora taxa known to occur in JTNP were undescribed when discovered.
Floral specialists, a case example in the subgenus Micranthophora
The subgenus Micranthophora specializes on a remarkable diversity of plants, especially considering how few species it contains. Some of these species, such as the Psorothamnus specialist Anthophora hololeuca (Figure 2), newly detected within JTNP in this study, will forage on just a few similar species. As a comparison, the genus Perdita consists mostly of oligoleges that will only collect pollen from a few closely related species or genera of plants. The genus Perdita includes 636 species but they only use 21 plant families, this works out to be roughly 0.03 plant families per species (Portman and Tepedino 2017). In contrast, Micranthophora contains 26 species that use 9 different plant families, i.e. 0.35 plant families per species (Orr et al. 2018) – a much higher ratio than seen in other groups of oligoleges. In part, this ratio is high because while the majority of Micranthophora are likely or confirmed Asteraceae specialists (17/26: 65%), the remaining nine species utilize plants from eight different families: Boraginaceae, Capparaceae, Cleomaceae, Fabaceae, Lamiaceae, Nyctaginaceae, Zygophyllaceae, and possibly Solanaceae. Efforts to determine host breadth are ongoing within this group of floral specialists, as the specific host plant(s) for many species remains uncertain, but one notable exception is discussed below.

Michael C. Orr
Anthophora abroniae and its floral host, Abronia villosa
The association of these two species appears to be the tightest of any Micranthophora, and it also ranks highly among bees in general, given the rarity of true monolecty (bees that visit only one host plant; Cane and Sipes 2006). The vast majority of female Anthophora abroniae specimens have been collected on Abronia villosa, Sand Verbena (Orr et al. 2018; Figure 4). Interestingly, females of this species have an unusually elongate mouthpart, called a galea, which is covered with mop-like hairs (Figure 4). This structure is used for manipulating flower parts and in this case is highly specialized to remove pollen from the narrow flowers of Abronia villosa.

Michael C. Orr
Figure 5. A male Anthophora petrophila grooms itself while perched on a stem by its mandibles, similar to how female Anthophora abroniae behave when moving pollen to their scopae (the place where pollen is stored on the legs).
These highly modified mouthparts enable Anthophora abroniae to very quickly and efficiently forage for pollen on this plant, though they would prove unwieldy and poorly-suited for most other plants. Consistently observed in the California deserts, including JTNP, females land on a cluster of Abronia villosa and move between flowers, quickly dipping their galea into each flower before moving to the next. As is common among many bees, the females will then groom pollen off of their hairy galea with their legs, either midflight or while holding onto the stem of a plant by their mandibles (Figure 5; Portman et al. 2019).
During this study, Anthophora abroniae was found at both Quail Springs Valley and Queen Valley, and it was collected prior to this study in the Pinto Basin. It was only collected or seen during spring, consistent with all but one record of this species (Orr et al. 2018), and consistent with the primary blooming period for Abronia villosa. Only three specimens were taken from JTNP because of how easily this species can be identified, thereby enabling better observation of its behavior. Over the course of approximately two hours of total observations at Quail Springs Valley, no Anthophora abroniae females were ever witnessed visiting plants other than Abronia villosa. In fact, the author has only ever witnessed a female Anthophora abroniae visiting an alternative plant (Palafoxia arida) once during >10 total hours of observation throughout the Desert Southwest. During the observation period in JTNP, males were repeatedly seen perching beside host plants and chasing each other away (along with other insects of adequate size).

Figure 6. All known collection sites of Anthophora abroniae (black circles). Anthophora abroniae was collected by the author at 10 sites, all of which had Abronia villosa in bloom (red stars). Joshua Tree National Park is shown as a green polygon; state and county boundaries are shown as gray lines. Scale 1:4,000,000.
Further supporting the close association of this bee and plant, the distribution of Anthophora abroniae fits well within that of the host plant (Figure 6; Tropicos 2017). Over the course of several years, the author has reviewed thousands of specimens from 30 institutions and visited numerous locations throughout the Desert Southwest where Abronia villosa occurs; Anthophora abroniae was never documented at a site that didn’t also have Abronia villosa in bloom (Figure 6). Most remarkable is a site near Bouse, AZ, where a single Abronia villosa plant was found (it was less than 8 cm in height with only one cluster of flowers) and even there, an Anthophora abroniae male was seen waiting beside the plant. With the many hours of observation and strong collection records now in place for Anthophora abroniae, this highly specialized relationship between these two species has only been reinforced and the groundwork has been laid for many interesting research opportunities. In the future, this system may prove useful for examining the ramifications of narrow host specialization in bees.
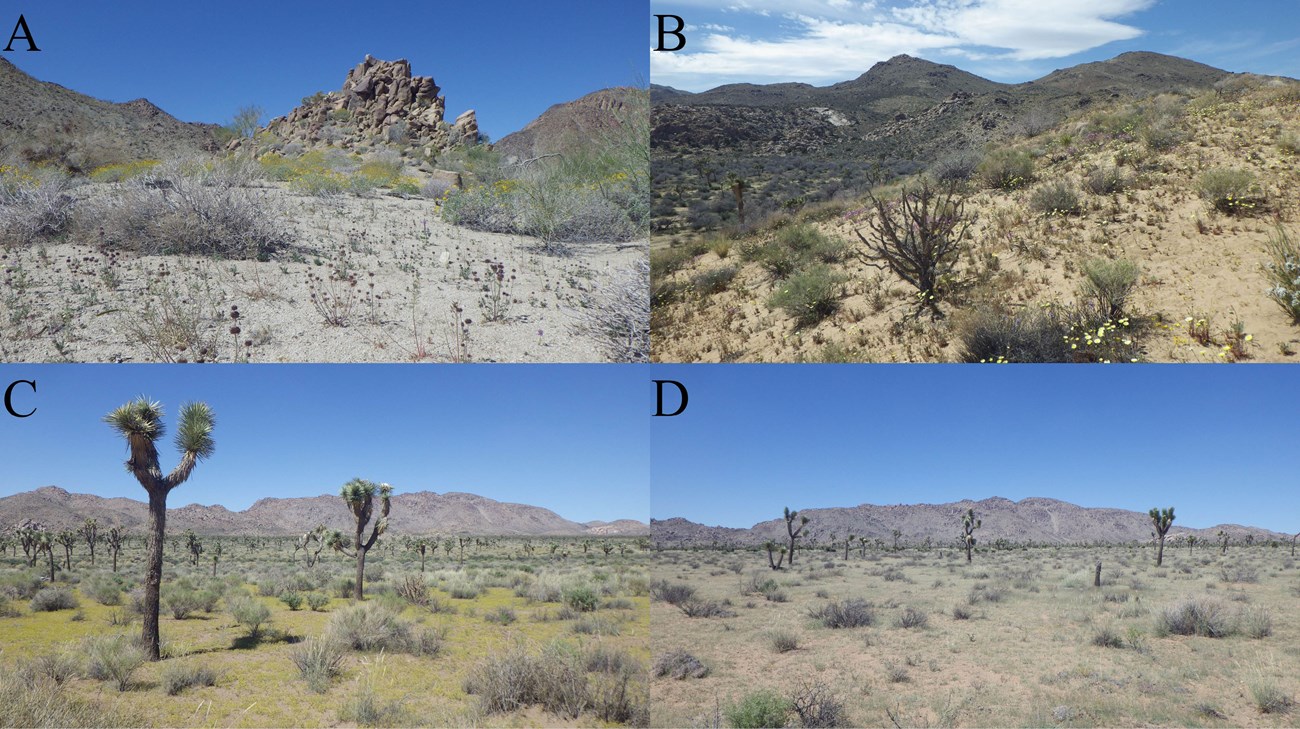
Figure 7. The best sampling localities from this study. A) Cottonwood Wash, where Chaenactis sp., Encelia farinosa, Larrea tridentata, Lupinus sp., Malacothrix sp., Parkinsonia florida, Phacelia campanularia, and Salvia columbariae were in bloom during spring 2016. B) Sandy bench along Quail Wash upon which Abronia villosa, Amsonia tomentosa, Baileya pleniradiata, Camissonia spp., Chaenactis spp., Cryptantha spp., Krameria bicolor, Malacothrix glabrata, Phacelia distans, and Salazaria mexicana were blooming in spring 2016. C) Queen Valley during fall (8/23/2015), with many flowering Pectis papposa, Abronia villosa, Chilopsis linearis, Encelia sp., Larrea tridentata, Nicolletia occidentalis, and Sphaeralcea ambigua were also in bloom. D) Different location in Queen Valley during fall (8/23/2015); this demonstrates the stochasticity of floral resource availability in the desert: the bloom is completely absent in this photo, which is less than one km away from the abundant bloom shown in (C).
Management implications
It is still too early to formally describe the structure of bee communities across the park, nor is it possible to accurately ascertain the species richness of bees found in JTNP. Without a doubt, further sampling throughout the various habitats in the park will prove valuable, as much of the park remains unexplored by entomologists (Figure 1). For example, in the higher elevations of the Little San Bernardino Mountains there are likely to be several genera commonly found in mild environments (e.g., Bombus, Dioxys, Protosmia), as well as other bee species that are generally found in the cismontane regions of southern California. In addition, many plant communities associated with the Sonoran Desert barely reach into the park along the southern and eastern boundaries, therefore providing an opportunity to document bees associated with these plant assemblages will likely be productive. Mesic habitats, such as palm oases, or ephemeral springs and washes are generally good sites for bee collecting, as these are also places where plants are more likely to bloom even in relatively dry years. As an example, Cottonwood Wash, Quail Springs Valley, and Queen Valley were all awash with bloom during at least one visit during this project and therefore were good places to document bee diversity (Figure 7). In extreme desert environments, where precipitation can be quite limited, areas with more consistent bloom are likely important reservoirs for bee species richness during dry years. However, some desert bees can wait multiple years to emerge, and, if widespread across many species, this trait could make consistent bloom less important to bee species richness in deserts (Danforth 1999, Orr et al. 2016). Any future sampling efforts should also focus on sampling during the summer and fall blooming periods, as this will undoubtedly add a number of taxa to the inventory, and perhaps even lead to additional species being discovered.
Counterintuitively, a plethora of flowers often makes for poor bee collecting. This is because a given year’s bee abundance is a consequence of the prior year’s floral resources, save for those species with multiple generations in a year (their second generations may be much larger than the first due to more resources within a year). This phenomenon was most evident on 26 March 2016 at the southern edge of JTNP, south of Cottonwood Wash. There, a wide array of flowers were in plentiful, peak bloom: Bebbia juncea, Chaenactis spp., Chylismia spp., Cryptantha spp., Encelia farinosa, Eriogonum spp., Eschscholzia spp., Larrea tridentata, Lupinus spp., Malacothrix glabrata, Mentzelia involucrata, Mentzelia nitens (likely), Nama demissa, Parkinsonia florida, Penstemon sp., Phacelia campanularia, Psorothamnus arborescens, and Salvia columbariae. Over the span of two hours at this site, however, a mere five bee specimens were collected, with only a few (<5) more observed. The combination of a poor bloom in spring 2015 (T. La Doux, pers. comm.) and many flowers in 2016 could dilute what few bees were present, while also decreasing their fidelity to any given plant; this would explain why there were so few bees despite the presence of many types of flowers.
An alternative explanation for this phenomenon is more harrowing. In recent years, climate change has been a central focus for studies of bee decline (Bartomeus et al. 2011, Forrest 2015, Potts et al. 2016, Settele et al. 2016). Phenological mismatches, where plants and pollinators no longer sync up in their seasonal activity, have been intensely explored in other habitats, but very few of these studies have occurred in deserts (Gerst and Venable 2017). Although results have been mixed, with many plants and pollinators responding to similar cues, some work has shown that plants may be impacted by mismatches (Bartomeus et al. 2011, Forrest 2015, Hegland et al. 2009, Rafferty et al. 2016). As many of these studies have focused on montane or relatively mild environments, it’s unclear what to expect in deserts, though one might expect pollinators that evolved in environments with high resource stochasticity to be better able to track their floral hosts under changed conditions. In the event that phenological mismatches are occurring, it is unclear how this issue might be alleviated.
Acknowledgements
I first thank Harold W. Ikerd and Tasha La Doux for their identifications and general support. Zachary M. Portman is also thanked for his identifications, as well as his input on Perdita biology. Terry Griswold, Vincent J. Tepedino, and Amber D. Tripodi are thanked for valuable discussions. Four anonymous reviewers are also thanked for their many improvements. This project was carried out with funding awarded to MCO by Joshua Tree National Park Association’s Robert Lee Graduate Student Research Program and The Community Foundation’s Desert Legacy Fund under permission from National Park Service study JOTR-00237, permit JOTR-2015-SCI-0006.

Michael C. Orr, PhD
As a postdoctoral researcher at the Chinese Academy of Sciences, Michael studies the systematics and evolutionary relationships of the bee group Anthophorini, with special focus on their biogeography and the evolution of various species traits. He focused on the desert bee group Anthophora for his PhD at Utah State, and during this time Michael’s sampling efforts contributed to a bee inventory for Joshua Tree National Park.
References
- Adondakis, S., and D.L. Venable. 2004. Dormancy and germination in a guild of Sonoran Desert annuals. Ecology 85(9):2582–2590.
- Bartomeus, I., J.S. Ascher, D. Wagner, B.N. Danforth, S. Colla, S. Kornbluth, and R Winfree. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences 108(51):20645–20649.
- Brooks, R.W. 1988. Systematics and Phylogeny of the Anthophorine Bees (Hymenoptera; Anthophoridae; Anthophorini). The University of Kansas Science Bulletin 53:436–575.
- Cane, J.H., and S. Sipes. 2006. Characterizing floral specialization by bees: analytical methods and a revised lexicon for oligolecty. Pages 99–122 in N.M. Waser and J. Ollerton, editors. Plant-pollinator interactions: from specialization to generalization. University of Chicago Press, Chicago, USA.
- Danforth, B.N. 1999. Emergence dynamics and bet hedging in a desert bee, Perdita portalis. Proceedings of the Royal Society of London B: Biological Sciences 266(1432):1985–1994.
- DL. 2017. DiscoverLife.org. http://www.discoverlife.org/ (Accessed 22–24 November 2017.)
- Forrest, J.R.K. 2015. Plant–pollinator interactions and phenological change: what can we learn about climate impacts from experiments and observations? Oikos 124:4–13.
- GBIF. 2017. Global Biodiversity Information Facility. https://www.gbif.org/ (Accessed 22–24 November 2017.)
- Gerst, K.L., and D.L. Venable. 2017. Phenology mediates reproductive success in the desert annual Chylismia brevipes. Mojave National Preserve Science Newsletter, April:8–12.
- Hegland, S.J., A. Nielsen, A. Lázaro, A.L. Bjerknes, and Ø. Totland. 2009. How does climate warming affect plant-pollinator interactions? Ecology letters 12(2):184–195.
- Hurd, P.D. 1957. Notes on the autumnal emergence of the vernal desert bee, Hesperapis fulvipes Crawford (Hymenoptera, Apoidea). Journal of the Kansas Entomological Society 30:10–10.
- Jurado, E., and M. Westoby. 1992. Germination biology of selected central Australian plants. Austral Ecology 17(3):341–348.
- Kemp, P.R. 1983. Phenological Patterns of Chihuahuan Desert Plants in Relation to the Timing of Water Availability. Journal of Ecology 71(2):427–436.
- Kopec, K., and L.A. Burd. 2017. Pollinators in Peril: A systematic status review of North American and Hawaiian native bees. Center for Biological Diversity.
- Litman, J.R., B.N. Danforth, C.D. Eardley, and C.J. Praz. 2011. Why do leafcutter bees cut leaves? New insights into the early evolution of bees. Proceedings of the Royal Society of London B: Biological Sciences 278:3593–3600.
- Michener, C.D. 1979. Biogeography of the bees. Annals of the Missouri botanical Garden 66(3):277–347.
- Michener, C.D. 2007. The Bees of the World. Second Edition. Johns Hopkins University Press, Baltimore, Maryland, USA.
- Minckley, R.L., J.H. Cane, and L. Kervin. 2000. Origins and ecological consequences of pollen specialization among desert bees. Proceedings of the Royal Society of London B: Biological Sciences 267:265–271.
- Nelson, R.A., and T.L. Griswold. 2015. The floral hosts and distribution of a supposed creosote bush specialist, Colletes stepheni Timberlake (Hymenoptera: Colletidae). Journal of Melittology 49:1–12.
- NPIC. 2017. USDA-ARS National Pollinating Insects Collection, Logan, UT. (Accessed 22 November 2017.)
- Olson, D.M., E. Dinerstein, E.D. Wikramanayake, N.D. Burgess, G.V.N. Powell, E.C. Underwood, J.A. D'Amico, I. Itoua, H.E. Strand, J.C. Morrison, C.J. Loucks, T.F. Allnutt, T.H. Ricketts, Y. Kura, J.F. Lamoreux, W.W. Wettengel, P. Hedao, and K.R. Kassem. (2001) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51:933–938.
- Orr, M.C., J.B. Koch, T.L. Griswold, and J.P. Pitts. (2014). Taxonomic utility of niche models in validating species concepts: A case study in Anthophora (Heliophila) (Hymenoptera:Apidae). Zootaxa 3846(3):411–429.
- Orr, M.C., T. Griswold, J.P. Pitts, and F.D. Parker. 2016. A new bee species that excavates sandstone nests. Current Biology 26(17):R792–R793.
- Orr, M.C., J.P. Pitts, and T. Griswold. (2018). Revision of the bee group Anthophora (Micranthophora) (Hymenoptera: Apidae), with notes on potential conservation concerns and a molecular phylogeny of the genus. Zootaxa 4511(1):1–193.
- Portman, Z.M., M.C. Orr, and T. Griswold. (2019). A review and updated classification of pollen gathering behavior in bees (Hymenoptera, Apoidea). Journal of Hymenoptera Research 71:171–208.
- Portman, Z.M., and V.J. Tepedino. 2017. Convergent evolution of pollen transport mode in two distantly related bee genera (Hymenoptera: Andrenidae and Melittidae). Apidologie 48(4):1–12.
- Potts, S.G., V. Imperatriz-Fonseca, H.T. Ngo, M.A. Aizen, J.C. Biesmeijer, T.D. Breeze, L.V. Dicks, L.A. Garibaldi, R. Hill, J. Settele, and A.J. Vanbergen. 2016. Safeguarding pollinators and their values to human well-being. Nature 540(7632):220–229.
- Rafferty, N.E., C.D. Bertelsen, and J.L. Bronstein. 2016. Later flowering is associated with a compressed flowering season and reduced reproductive output in an early season floral resource. Oikos 125(6):821–828.
- Ritchie, A.D., R. Ruppel, and S. Jha. 2016. Generalist Behavior Describes Pollen Foraging for Perceived Oligolectic and Polylectic Bees. Environmental Entomology 45(4):909–919.
- Settele, J., J. Bishop, and S.G. Potts. 2016. Climate change impacts on pollination. Nature Plants 2:16092.
- Tevis, L. 1958. Germination and Growth of Ephemerals Induced by Sprinkling a Sandy Desert. Ecology 39(4):681–688.
- Timberlake, P.H. 1956. A revisional study of the bees of the genus Perdita F. Smith, with special reference to the fauna of the Pacific Coast (Hymenoptera, Apoidea) Part II. University of California Press 11(5):247–350.
- Tropicos. 2017. http://www.tropicos.org/ (Accessed 23 November 2017.)
- Wcislo, W.T., and J.H. Cane. 1996. Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annual Review of Entomology 41:257–286.
- Wilson, J.S., J.P. Pitts, and C.D. von Dohlen. 2009. Lack of variation in nuclear genes among isolated populations of the sand dune restricted bee Colletes stepheni (Hymenoptera: Colletidae). Journal of the Kansas Entomological Society 82(4):316–320.
