Wikamedia
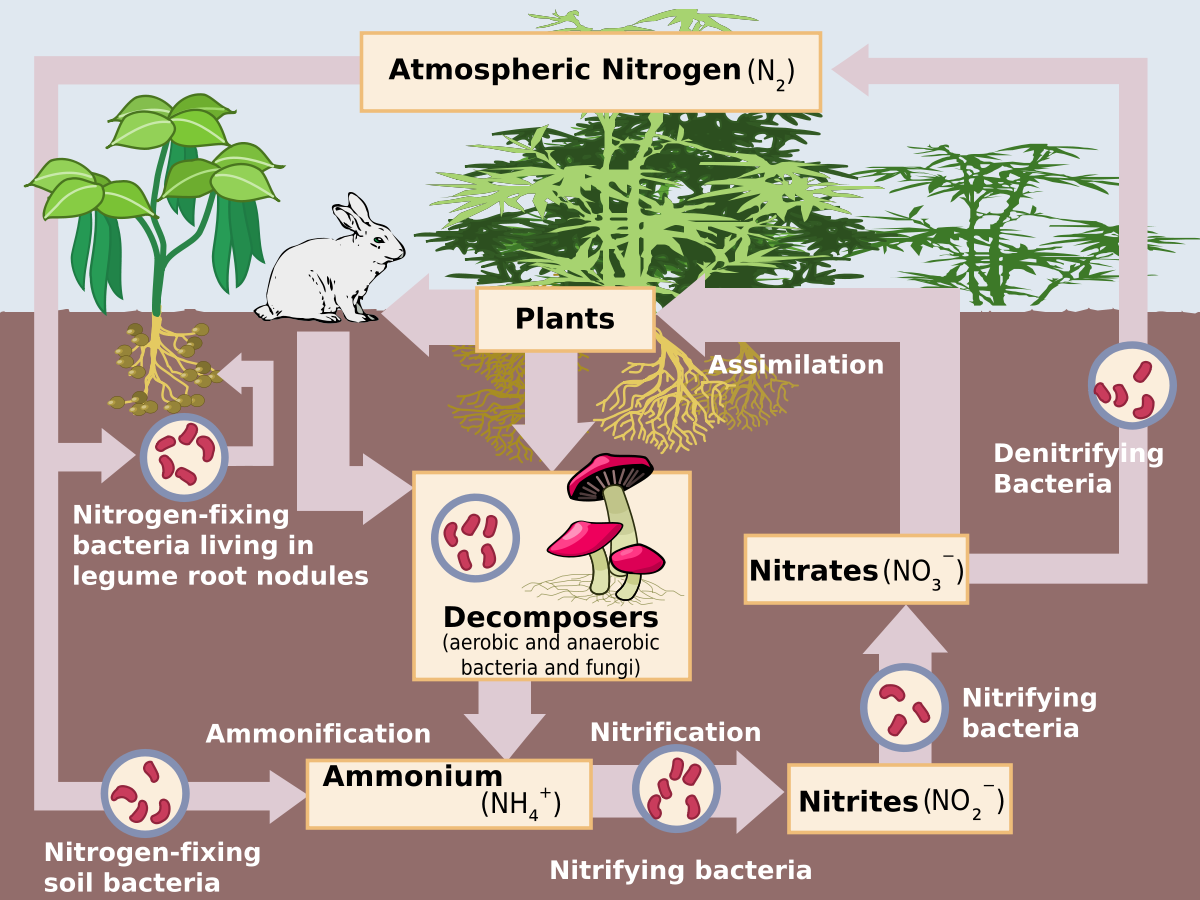
Nitrogen (N) makes up 78% of the Earth's atmosphere, but this form of nitrogen (N2) is inaccessible to most plants and animals. Animals, plants, and humans all need N to exist. It is the building block of proteins which make up our muscles and enzymes which fuel bodily functions, it is the backbone of our DNA, and in plants it is a major component of chlorophyll, which allows plants to photosynthesize (transform sunlight energy and carbon dioxide into sugars which fuels them and us).
The N cycle is complex and sensitive to change. For much of earth's history it has been kept in a delicate balance. Most N exists in an organic form, inaccessible to organisms. Biological activities such as microbial fixation transform organic N into reactive N which is then used by soil organisms and plants.
NPS, 2014
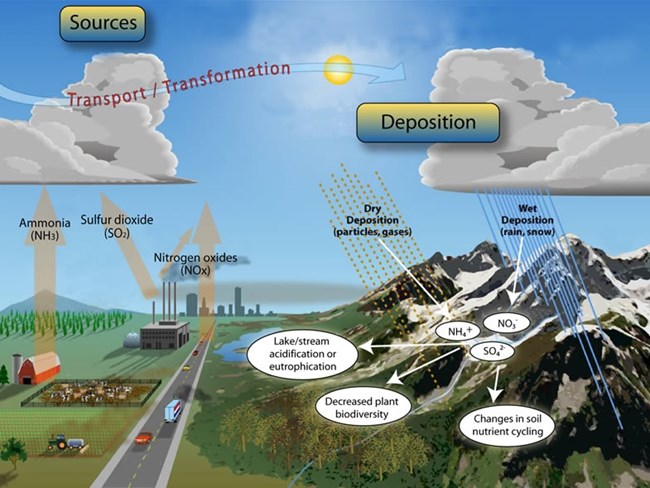
Human activities are increasing the load of reactive N in the environment. Anthropogenic sources of N include nitrogen oxides (NOx) and ammonia (NH3) which stem from the burning of fossil fuels and agricultural practices such as waste management and fertilizer application, respectively.
Once these pollutants enter the atmosphere as gases or microscopic particles they can fall back down to the earth as particles (dry deposition) or undergo chemical reactions forming other compounds (nitrate (NO3-), and nitrite (NO2-) and ammonium (NH4+)) which can be transported to the earth’s surface via rain and/or snow referred to as wet deposition.
Just as we fertilize crops to help them grow, human additions of reactive N acts like fertilizer on ecosystems. While we choose where we apply fertilizer this reactive N deposition rains down indiscriminately on natural ecosystems.
Natural systems have evolved under species levels of N and these large fluxes of N into ecosystems can disrupt the delicate balance. For example:
- Algal blooms on lakes
- Increases of invasive plants species
- Loss of native plant species
- Changes in soil chemistry that lead to leaching of N into the groundwater
NPS
Sometimes you can have too much of a good thing for example: sunshine. We need it so we can produce vitamin D to keep our bones strong and to warm our skin on a cold day, but too much sunlight and we get sunburnt.
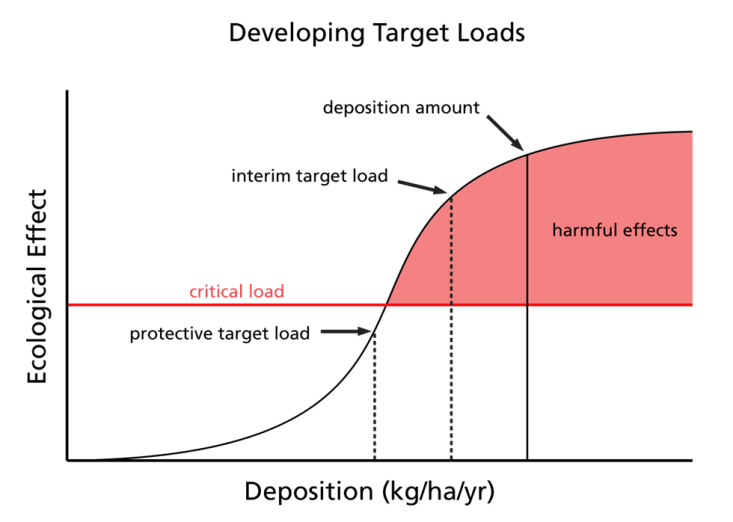
Ecosystems need N to function but can become saturated resulting in negative consequences. Scientists and resources managers are determining the threshold at which organisms and ecosystems shift from just enough to too much, this level is called the critical load. A critical load is specifically defined as the threshold of deposition below which specified harmful ecological effects do not occur (Porter et al. 2005).
Critical loads are being developed in the U.S. and are expressed as kilograms of N per hectare per year (kg N/ha/yr). Scientists calculate critical loads using ecosystem modeling, field observations, and experiments. Specific species and ecosystems are more sensitive to N than others and thus serve as ideal biological indicators of change, for example: lichens and phytoplankton in lakes, soil chemistry, water chemistry, herbaceous plant communities, and forest communities.
Nitrogen Deposition Research
Last updated: July 6, 2018
