Last updated: March 15, 2024
Article
Unique Coral Community in the Mangroves of Hurricane Hole
Abstract:
Corals do not typically thrive in mangrove environments. However, corals are growing onand near the prop roots of red mangrove trees in Hurricane Hole, an area within the Virgin Islands Coral Reef National Monument under the protection of the US National Park Service in St. John, US Virgin Islands. This review summarizes current knowledge of the remarkable biodiversity of this area. Over 30 scleractinian coral species, about the same number as documented to date from nearby coral reefs, grow here. No other mangrove ecosystems in the Caribbean are known to have so many coral species. This area may be a refuge from changing climate, as these corals weathered the severe thermal stress and subsequent disease outbreak that caused major coral loss on the island’s coral reefs in 2005 and 2006. Shading by the red mangrove trees reduces the stress that leads to coral bleaching. Seawater temperatures in these mangroves are more variable than those on the reefs, and some studies have shown that this variability results in corals with a greater resistance to higher temperatures. The diversity of sponges and fish is also high, and a new genus of serpulid worm was recently described. Continuing research may lead to the discovery of more new species.
Keywords: corals; mangroves; climate change refuge; thermal stress; US Virgin Islands

Introduction
For hundreds of years, four bays within Hurricane Hole, St. John, US Virgin Islands, have been a refuge for boats during major storms (Figure 1). Hurricane Hole is a beautiful seascape with coral reefs, mangroves, seagrass beds and a remarkable biodiversity in corals, fishes, sponges and other organisms. This area is part of the Virgin Islands Coral Reef National Monument established in 2001 by President Clinton through a Presidential Proclamation. The particular biological and physical oceanographic conditions within these four mangrove-lined bays within Hurricane Hole (which range in size from 0.06 to 0.11 km2) appear to be providing a refuge for corals at a time of changing climate [1], (Figure 2 a, b, c, d). Corals and the reefs they create are at risk from numerous human activities around the world [2].
Corals do not usually thrive among mangroves because of the typically turbid and low (and sometimes high) salinity waters. Here in Hurricane Hole there are no permanent streams entering the bays, with the only freshwater coming from rainfall and possibly from groundwater. The mangroves provide shade, attenuating more than 70% of the photosynthetically active radiation (PAR) [1]. In addition, the proximity of seagrass beds, mangroves, and patch reefs, and the particular hydrographic conditions and biological influences of these habitats on seawater chemistry, generate chemical conditions that buffer against lower pH (ocean acidification). Ocean acidification reduces the rates at which calcifiers such as corals and coralline algae grow and causes carbonate sediment dissolution [3].
significance of the mangroves in Hurricane Hole, particularly their role as a nursery, was
highlighted (see text box). However, it was not until 2009 that the extraordinary diversity of the corals was recognized. Active management by the US National Park Service, including the installation of a storm mooring system, is no doubt helping to protect this diverse ecosystem. The secure mooring system decreases the likelihood that boats will break free or drag their anchors and go up into the mangroves.
1.1. Stony Corals
Remarkably, over 30 species of scleractinian corals grow on or close to the prop roots of the red mangroves along the shores of three of the bays (Princess Bay, Otter Creek, and Water Creek). (Table 1, Figure 3). Only a few species found on nearby coral reefs (such as Mussa angulosa, and Madracis spp.) have not been seen in these bays, and a few have been observed in the bays but not listed as occurring on the reefs (see Table 1). No other mangrove ecosystems in the Caribbean are known to harbor so many coral species. The species present include major reef framework building species such as brain corals (Colpophyllia natans; Diploria labyrinthiformis) and star corals (Orbicella spp.), as well as species with smaller colonies such as Porites astreoides. Corals in the genus Orbicella are some of the most abundant corals on the reefs around St. John and in the mangroves. Surprisingly, some corals that are quite rare on the coral reefs and more often found in deeper water, such as Mycetophyllia spp. and Scolymia spp., are found growing in the shade of the mangroves. Some corals also grow on patch reefs in deeper water in the bays. The sides of the bays slope to the sea floor to a depth of about 5 m and then the floor slopes more gradually to the centers of the bays. Corals are more numerous near the entrances to the bays, but, surprisingly, can also be found in low numbers in the calmer, siltier portions at the head of the bays. The middle of the bays where depths reach 10 to 14 m can be turbid. Generally the water is quite clear right along the shore likely due to filter feeding organisms like tunicates, sponges and tree oysters which grow there [4,5]. Six of the seven Caribbean coral species listed as threatened under the US Endangered Species Act (Mycetophyllia ferox has not been reported) are found in Hurricane Hole, with four of them in these mangrove-fringed bays (Orbicella annularis, O. faveolata, O. franksi, Dendrogyra cylindrus). Three small colonies of one of the other listed species, Acropora palmata, were also observed to have settled there but did not survive to grow into larger colonies. Acropora cervicornis has not been found.
Figure 3. Stony corals in the mangrove-lined bays of Hurricane Hole. (a) a colony of Orbicella annularis; (b) a colony of Orbicella faveolata with a small Orbicella sp. recruit visible on the rock just in front of it; (c) Colpophyllia natans (foreground) and Orbicella faveolata; (d) Siderastrea siderea; (e) Undaria agaricites and Millepora alcicornis; (f) Poritesfurcata; (g) Porites astreoides; (h) Diploria labyrinthiformis and Orbicella annularis surrounded by Porites porites; (i) Eusmilia fastigiata; (j) Scolymia sp.; (k) Mycetophyllia aliciae with Porites astreoides in the background.
These shallow, mangrove-associated coral communities differ from “true” or framework” coral reefs, which are rigid, topographically complex structures developed from carbonate accretion by corals and other cementing and calcifying organisms and the product of biological and geological processes [6,7]. The corals in the mangroves are growing on prop roots or hard non-limestone substrata nearby, and are not currently depositing layers of limestone that accrete into structurally complex reefs.


As water temperatures cooled in the fall of 2005, bleached coral colonies gradually regained their normal coloration. However, an outbreak of white plague disease peaked 2 to 6 months (at five different locations) after the peak in bleaching [8]. The disease affected all coral species and resulted in an average decline of 60% in living coral cover by the end of 2007. The decline was associated with partial and complete mortality of coral colonies. Orbicella spp. were affected more than other species but remained the most abundant following the outbreak. Corals on reefs in the other US Virgin Islands, British Virgin Islands, and Puerto Rico also bleached severely in 2005 with subsequent disease outbreaks.
The relationships among high sea water temperatures, bleaching, and diseases are complex. Bleaching episodes are not always followed by disease outbreaks, which are sometimes not preceded by bleaching. However, compelling evidence exists that many diseases are associated with unusually warm temperatures, and ocean temperatures are predicted to continue increasing in the future. Diseases are increasing in prevalence and becoming more severe. They are challenging to study and few pathogens or causes have been identified conclusively [11].
The significance of the mangrove-lined bays as habitats for corals was not recognized until 2009, and no baseline studies of corals in the mangroves in Hurricane Hole were undertaken before or during the 2005 to 2007 bleaching/disease event. However, the sizes (over 0.5 to 0.75 m across) of many colonies of the major reef-building corals in the mangroves and their generally intact condition with few dead portions indicate that they were present in 2005 and survived the bleaching/disease episode. Some of the largest and most numerous corals there are colonies of boulder brain corals (Colpophyllia natans) and grooved brain corals (Diploria labyrinthiformis). Both of these species seem to be relatively more abundant in the mangroves than on the reefs nearby. Although no rigorous comparison has been made, most C. natans in the mangroves appear to be in better condition (i.e., dead portions are rare) than on the reefs (Figure 5).

In 2010, another less severe bleaching episode occurred allowing comparison of the responses of these two species. Successive observations (and photographs) of colonies in the mangroves documented much less bleaching in Colpophyllia natans than in Diploria labyrinthiformis (Figure 6), and only low amounts of complete mortality (0 to 7.1%, respectively). Most colonies recovered completely [12]. Because of the low abundance of these species on the reef transects, no rigorous comparison could be made of the severity of or responses to thermal stress between these two habitats. Bleaching results from high temperatures and high irradiance [13]. In 2010, the temperatures in the mangroves as recorded by in situ thermometers at a depth of less than 1 m reached both higher and lower values than those recorded on the reefs at a depth of about 10 m [1]. At first glance, the mangroves would not seem to be offering a refuge from higher temperatures. However, variable temperatures have been associated with corals that have greater resistance to bleaching in some studies [14]. Research is underway to characterize the genotypes and microbial communities associated with colonies from Hurricane Hole to see if they provide clues to their responses to temperature increases or other parameters such as irradiance levels [15].
1.2 Fishes
About 80 species of fish have been recorded from Hurricane Hole [16] (Table 2, Figure 7). Gray Snappers (Lutjanus griseus) are among the largest and most abundant. Large parrotfishes are noticeably scarce. Queen Angelfishes (Holocanthus ciliarus) are conspicuous, perhaps because they feed on the numerous sponges. Schools of grunts are often seen among the roots, and the three species of trunkfishes are present.
Table 2. Fish species in Hurricane Hole, based on Boulon (1992) [16] with recent additions. Source of names is Fishbase or World Register of Marine Species.
A few very rare fish have also been spotted in the mangroves. In 2010, several individuals of the Sargassum Frogfish (Histrio histrio) were observed in seaweed growing on the prop roots, the first report of this species living in association with attached algae [17]. They are normally found in floating mats of Sargassum seaweed. Likewise, in 2015, a Unicorn Filefish (Aluterus monoceros) was observed and photographed in Otter Creek. Either this fish, or another one of the same species, was observed in May 2013 in another bay within Hurricane Hole. This is the rarest of the seven species of filefishes in the Caribbean, and it has not been reported before from St. John, or from nearby St. Croix [18] or St. Thomas. Nor was it seen around St. John between 2000 and 2014 in 1694 surveys conducted by scientists with the National Oceanic and Atmospheric Administration.
Lionfish, native to the Indo-Pacific, were first observed in the mangroves in Hurricane Hole in early 2010 [19]. They have been spreading throughout the Caribbean since about 1992 [20]. They prey on juvenile and adult reef fishes [21]. A group of local divers have been removing these fish around St. John.
1.3. An Invasive Seagrass
Another invasive species, the seagrass Halophila stipulacea, originally from the Red Sea and Indian Ocean, was first seen in Hurricane Hole in 2012. This seagrass has been colonizing more and more areas progressing north through the Caribbean since it first became established in Grenada in 2002 [22,23]. It has reached Puerto Rico and will likely become established in Florida. Unlike the native Caribbean seagrasses Thalassia testudinum and Syringodium filiforme, entire plants of this species, rather than just blades, float in the currents, and they are well-adapted to settle and grow on bare sand. The ecological effects of this species are not yet understood (Figure 8).
Figure 8.


Left image
(a) The invasive seagrass Halophila stipulacea growing with the native seagrass Thalassia testudinum.
Credit: Caroline Rogers
Right image
(b) The invasive seagrass Halophila stipulacea floats with its roots and rhizomes attached.
Credit: Caroline Rogers

1.4. Sponges
About 60 species of sponges grow in the mangrove-lined bays within Hurricane Hole [24]. Many are more characteristic of reefs than of mangroves. The Cushion Sea Star (Oreaster reticulatus) feeds on some of these sponges, and the “chicken liver” sponge (Chondrilla caribensis), which grows over many of the rocks in the area, is one of the Hawksbill turtle’s favorite foods [25]. Several Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) sea turtles have been seen in Hurricane Hole (Figure 9).
1.5. Other Inhabitants
Many other organisms live in Hurricane Hole, including octocorals, anemones, jellyfish, crabs, shrimp, lobsters, tunicates, bryozoans, octopuses, conchs, starfish, and algae. Conspicuous examples include the species illustrated in Figure 10.
Figure 10.
(a) Banded Coral Shrimp (Stenopus hispidus); (b) Spiny lobster (Panulirus argus); (c) Channel clinging crab (Damithrax spinosissimus); (d) Giant Caribbean Anemone (Condylactis gigantea); (e) Beaded Anemone (Epicystis crucifer); (f) Common Octopus (Octopus vulgaris); (g) Queen Conch (Lobatus gigas); (h) Cushion Star (Oreaster reticulatus); (i) Upside Down Jellyfish (Cassiopea sp.); (j) Bryozoan; (k) Tunicate.
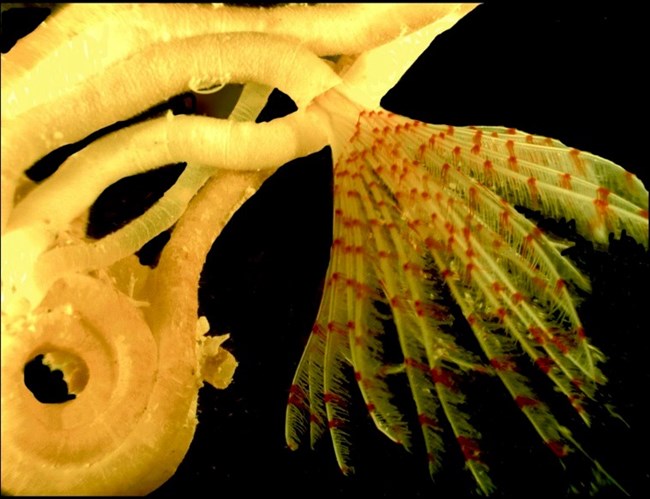
1.6. Discovery of a New Genus
No comprehensive inventory of the mangrove-fringed bays within Hurricane Hole has been compiled. It is likely that more species remain to be discovered. For example, Dr. Nancy Prentiss (University of Maine) recently described a new genus of serpulid worm, Turbocavus secretus [26] .
(Figure 11). The genus name, Turbocavus (turbo = hurricane in Latin; cavus refers to cavity or hidden tube), refers to the location in which it was found. The worm also builds a calcareous tube that is cyclonic in shape, hence the name “turbo.” The species name reflects that it was “secretly hidden” (secretus) in Hurricane Hole.
2. Conclusions
It is surprising that over 30 coral species are found in the mangroves of Hurricane Hole. No other Caribbean mangrove ecosystem is known to have such a high diversity of corals. Coral colonies are generally in good condition, and recruits of several species have settled on the mangrove prop roots or on nearby substrata. How will this community respond to increasing sea temperatures and sea level rise? How is Hurricane Hole linked to the surrounding seascape through connectivity of the larvae of fish, corals and other reef organisms? The area offers outstanding possibilities for further research. The particular combination of biological and physical environmental factors and the high diversity of coral and other species may make this ecosystem more resistant and more resilient to future local, regional and global stressors [1,27].
Acknowledgments: My thanks to Simon Pittman (NOAA) for creating Figure 1 and to Nancy Prentiss for the photograph of the new serpulid species. Chris Jeffrey and Sarah Hile (NOAA) helped with the list of fish species. USGS scientists Ilsa Kuffner and Kristen Hart, NPS scientists Jeff Miller and Lee Richter, and Rafe Boulon, plus two anonymous reviewers, made very helpful suggestions on the paper. Thanks to all. Conflicts of Interest: The author declares no conflict of interest.
You can download the article here.
References
1. Yates, K.; Rogers, C.; Herlan, J.; Brooks, G.; Smiley, N.; Larson, R. Mangrove habitats provide refuge from climate change for reef-building corals. Biogeosci. Discuss. 2014, 11, 5053–5088. [CrossRef]
2. Burke, L.; Reytar, K.; Spalding, M.D.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011.
3. Kleypas, J.A.; Yates, K.K. Coral reefs and ocean acidification. Oceanography 2009, 22, 108–117. [CrossRef] 4. Riisgard, H.U.; Larsen, P.S. Filter-feeding in marine macro-invertebrates: Pump characteristics, modelling and energy cost. Biol. Rev. Camb. Philos. Soc. 1995, 70, 67–106. [CrossRef] [PubMed]
5. Newell, R. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve Molluscs: A review. J. Shellfish Res. 2004, 23, 51–61.
6. Hubbard, D. Reefs as dynamic systems. In Life and Death of Coral Reefs; Birkeland, C., Ed.; Chapman & Hall: New York, NY, USA, 1997; pp. 43–67.
7. Rogers, C.; Miller, J. Measuring, interpreting, and responding to changes in coral reefs: A challenge for biologists, geologists, and managers. In Coral Reefs at the Crossroads; Hubbard, D., Rogers, C., Lipps, J., Stanley, G., Jr., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 277–292.
8. Miller, J.; Muller, E.; Rogers, C.; Waara, R.; Atkinson, A.; Whelan, K.R.T.; Patterson, M.; Witcher, B. Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 2009, 28, 925–937. [CrossRef]
9. Eakin, C.M.; Morgan, J.A.; Heron, S.F.; Smith, T.B.; Liu, G.; Alvarez-Filip, L.; Baca, B.; Bartels, E.; Bastidas, C.; Bouchon, C. Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PLoS ONE 2010, 5, e13969. [CrossRef] [PubMed]
10. Hoegh-Gulberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [CrossRef]
11. Weil, E.; Rogers, C.S. Coral Reef Diseases in the Atlantic-Caribbean. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 465–491.
12. Rogers, C.; Herlan, J. Life on the Edge: Corals in Mangroves and Climate Change. In Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9–13 July 2012; p. 5.
13. Jokiel, P.; Coles, S. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 1990, 8, 155–162. [CrossRef]
14. Woesik, R.; Houk, P.; Isechal, A.L.; Idechong, J.W.; Victor, S.Y. Climate-change refugia in the sheltered bays of Palau: Analogs of future reefs. Ecol. Evol. 2012, 2, 2474–2484. [CrossRef] PubMed]
15. Muller, E.; Mote Marine Laboratory, Sarasota, FL, USA. Personal communication, 2016.
16. Boulon, R.H. Use of Mangrove Prop Root Habitats by Fish in the Northern U.S. Virgin Islands. In Proceedings of the Gulf and Caribbean Fishery Institute, St. Thomas, VI, USA, 10–14 May 1990; pp. 189–204.
17. Rogers, C.; Pietsch, T.; Randall, J.; Arnold, R. The Sargassum Frogfish (Histrio histrio Linnaeus) observed in mangroves in St. John, US Virgin Islands. Coral Reefs 2010, 29, 577. [CrossRef]
18. Smith-Vaniz, W.F.; Jelks, H.L.; Rocha, L.A. Relevance of cryptic fishes in biodiversity assessments: A case study at Buck Island Reef National Monument, St. Croix. Bull. Mar. Sci. 2006, 79, 17–48.
19. Herlan, J.; Catholic University of the North, Antofagasta, Chile. Personal communication, 2010.
20. Schofield, P.J. Geographic extent and chronology of the invasion of non-native lionfish (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea. Aquat. Invasions 2009, 4, 473–479. [CrossRef]
21. Albins, M.A.; Hixon, M.A. Worst case scenario: Potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ. Biol. Fishes 2013, 96, 1151–1157. [CrossRef]
22. Rogers, C.; Willette, D.; Miller, J. Rapidly spreading seagrass invades the Caribbean with unknown ecological consequences. Front. Ecol. Environ. 2014, 12, 546–547. [CrossRef]
23. Willette, D.A.; Chalifour, J.; Debrot, A.D.; Engel, M.S.; Miller, J.; Oxenford, H.A.; Short, F.T.; Steiner, S.C.; Védie, F. Continued expansion of the trans-Atlantic invasive marine angiosperm Halophila stipulacea in the Eastern Caribbean. Aquat. Bot. 2014, 112, 98–102. [CrossRef]
24. Wulff, J.; Florida State University, Tallahassee, FL, USA. Personal communication, 2011.
25. Meylan, A. Spongivory in hawksbill turtles: A diet of glass. Science 1988, 239, 393–395. [CrossRef] [PubMed]
26. Prentiss, N.K.; Vasileiadou, K.; Faulwetter, S.; Arvanitidis, C.; Ten Hove, H.A. A new genus and species of Serpulidae (Annelida, Polychaeta, Sabellida) from the Caribbean Sea. Zootaxa 2014, 3900, 204. [CrossRef] [PubMed]
27. Rogers, C. Coral reef resilience through biodiversity. ISRN Oceanogr. 2013. [CrossRef]
